Federal health officials widened the scope of the infant botulism outbreak linked to ByHeart formula, now covering all illnesses since the company began production in March 2022.
Expanded Outbreak
The U.S. Food and Drug Administration said investigators “cannot rule out the possibility that contamination might have affected all ByHeart formula products ever made.” The outbreak now includes at least 51 infants in 19 states. The most recent illness was reported on Dec. 1, and no deaths have been reported. The outbreak was first announced on Nov. 8.
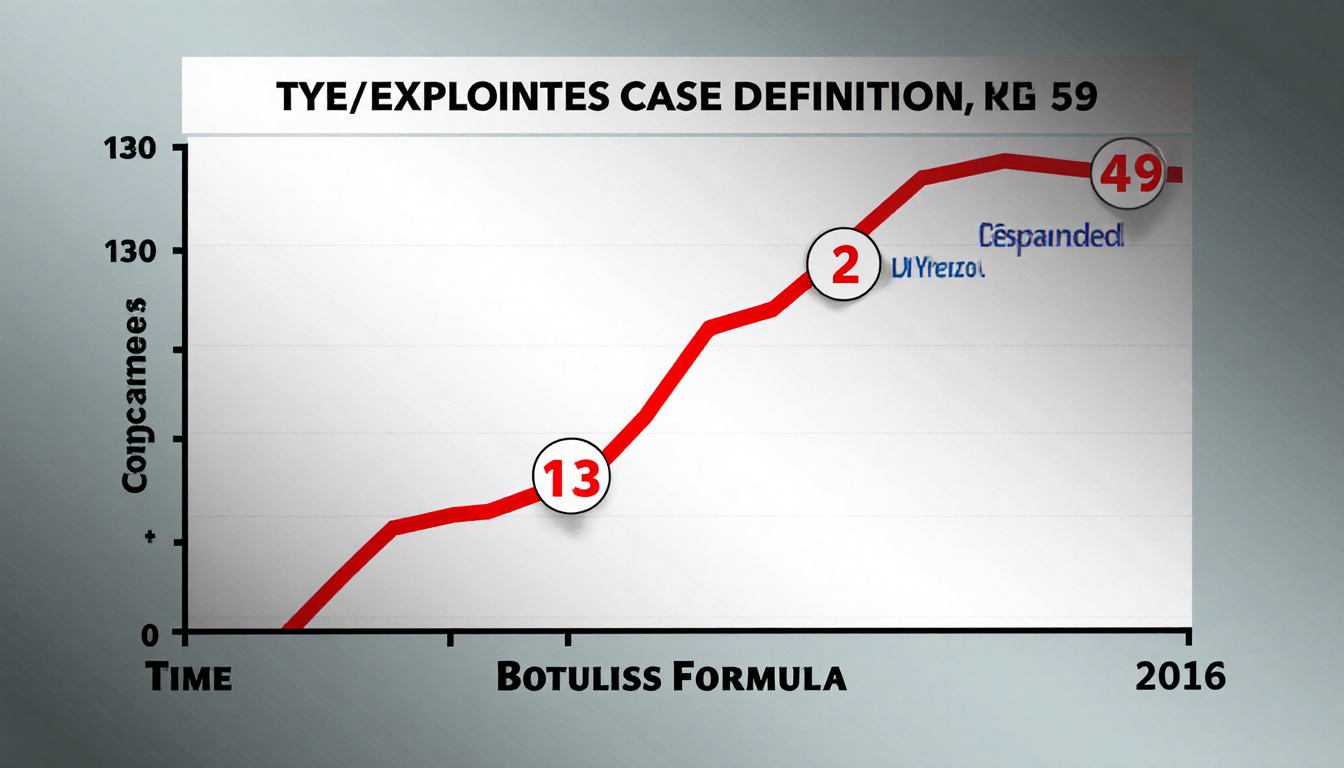
New Case Definition
The U.S. Centers for Disease Control and Prevention added a new case definition that includes any infant with botulism who was exposed to ByHeart formula at any time since the product’s release. Previously, officials had reported 39 suspected or confirmed cases in 18 states since August. The expanded definition added 12 more cases, including two that overlapped the original timeline and ten that occurred from Dec. 2023 through July 2025.
ByHeart Recall and Production
ByHeart, a New York‑based manufacturer of organic infant formula founded in 2016, recalled all its products sold in the U.S. on Nov. 11. The company, which accounts for about 1% of the U.S. infant formula market, had been selling roughly 200,000 cans each month.
Family Voices
Andi Galindo, 36, of Redondo Beach, California, said her 5‑week‑old daughter Rowan was hospitalized in December 2023 with infant botulism after drinking the formula. She added, “That’s a hard one. If there is proof that there were issues with their manufacturing and their plant all the way back from the beginning, that is a problem and they really need to be held accountable.”
Amy Mazziotti, 43, of Burbank, California, said her then‑5‑month‑old son Hank fell ill and was treated for botulism in March, weeks after he began drinking ByHeart. She said, “I’ve known in my gut from the beginning that ByHeart was the reason Hank got sick, and to see that these cases are now part of the investigation brings me to tears — a mix of relief, gratitude and hope that the truth is finally being recognized.”
Investigation and Testing
The FDA sent inspectors last month to ByHeart plants in Allerton, Iowa, and Portland, Oregon, where the formula is produced and packaged. The agency has released no results from those inspections. Independent laboratory tests had shown that 36 samples from three different lots contained the type of bacteria that can cause infant botulism. ByHeart’s website last month stated, “We cannot rule out the risk that all ByHeart formula across all product lots may have been contaminated.”
Dr. Jennifer Cope, a CDC scientist leading the investigation, said, “It looks like the contamination appeared to persist across all production runs, different lots, different raw material lots. They couldn’t isolate it to specific lots from a certain time period.”
Inspection documents revealed ByHeart had a history of contamination problems. In 2022, the company recalled five batches after a sample at a packaging plant tested positive for cronobacter sakazakii. In 2023, the FDA issued a warning letter detailing “areas that still require corrective actions.” A plant in Reading, Pennsylvania, was shut down in 2023 just before FDA inspectors found mold, water leaks and insects.
Infant Botulism Overview
Infant botulism is a rare disease that affects fewer than 200 babies in the U.S. each year. It is caused when infants ingest botulism bacteria that produce spores that germinate in the intestines, creating a toxin that affects the nervous system. Babies are vulnerable until about age 1 because their gut microbiomes are not mature enough to fight the toxin.
Symptoms can take up to 30 days to develop and include constipation, poor feeding, loss of head control, drooping eyelids and a flat facial expression. Babies may feel “floppy” and can have problems swallowing or breathing.
The sole treatment for infant botulism is BabyBIG, an IV medication made from pooled blood plasma of adults immunized against botulism. California’s infant botulism program developed the product and is the sole source worldwide. Dr. Sharon Nachman, an expert in pediatric infectious disease at Stony Brook Children’s Hospital, wrote an email saying, “The risk to the infant is ongoing and the family should not be using this formula after it was recalled.”
Legal Actions
Families of several babies treated for botulism after drinking ByHeart formula have sued the company. Lawsuits filed in federal courts allege that the formula was defective and that ByHeart was negligent in selling it. They seek financial payment for medical bills, emotional distress and other harm.
Key Takeaways
- The outbreak now covers 51 infants in 19 states, expanding to all illnesses since March 2022.
- No deaths have been reported, and the recall began on Nov. 11.
- Independent tests and inspections suggest contamination may have persisted across all production runs.
The expanded investigation underscores the seriousness of potential contamination in infant formula and the ongoing effort to protect vulnerable babies.




